Participation rate
Participation rate tells us what proportion of people who are eligible for colorectal cancer screening have been screened within guidelines. Eligibility and recruitment methods for colorectal cancer screening are different in each province and territory. There are many different ways to measure the participation of the population in colorectal cancer screening, and we report on 3 key measures:
- Screen-eligible population-based program participation rate
- Screening program participation rate (among those invited to screen)
- Percentage overdue for colorectal cancer screening
1. Screen-eligible population-based program participation rate
Why is this important? To see the benefits of colorectal cancer screening, including early detection and decreased incidence and mortality, eligible people need to participate in screening. This indicator tells us how many people in the eligible age group are participating in the colorectal cancer screening program.
Indicator definition: The percentage of eligible people who successfully completed at least one fecal test between January 1, 2017 and June 30, 2019.
Target: ≥ 60% of eligible people screened within 2 years
Indicator calculation:
Numerator: Number of people who successfully completed one or more fecal test in the program in a 30-month period.
Denominator: Number of people who are eligible for screening within the measurement timeframe.
Additional considerations: A 30-month period is used to allow people who become part of the target age group near the end of the measurement timeframe a grace period of six months after their 2-year anniversary date in which to be screened.
How does 2017/18 compare to 2013/14? Compared with data in the colorectal cancer screening report for 2013-14 when program participation rates ranged from 8.6% to 53.0%, programmatic participation rates have increased overall in 2017/18.
COVID-19 considerations
![]() Participation rates declined during the COVID-19 pandemic as screening programs paused services and recruitment, and many people delayed screening.1 Participation rate is an indicator that colorectal cancer screening programs will need to monitor closely during the COVID-19 pandemic recovery phase to increase participation rates back to pre-pandemic levels. It will be important to develop strategies to ensure that people who are overdue for screening due to the COVID-19 pandemic are able to receive timely screening.
Participation rates declined during the COVID-19 pandemic as screening programs paused services and recruitment, and many people delayed screening.1 Participation rate is an indicator that colorectal cancer screening programs will need to monitor closely during the COVID-19 pandemic recovery phase to increase participation rates back to pre-pandemic levels. It will be important to develop strategies to ensure that people who are overdue for screening due to the COVID-19 pandemic are able to receive timely screening.
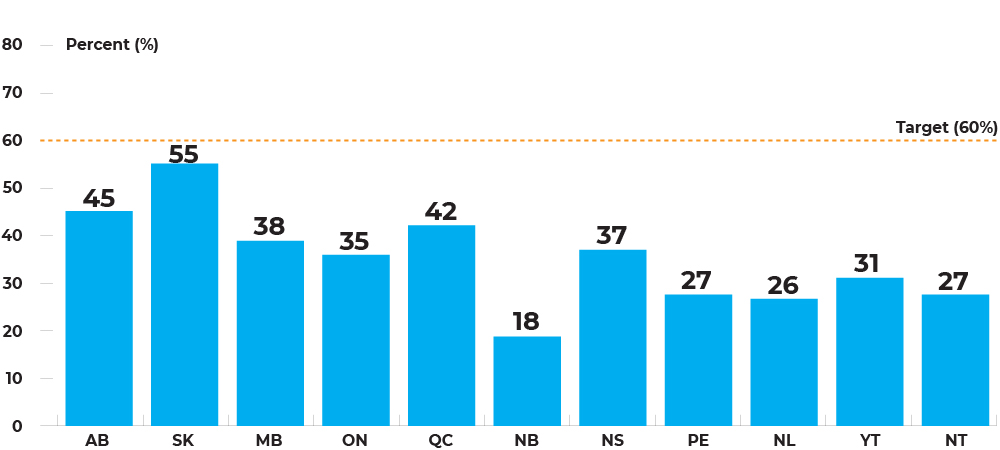
Population-based participation rates for fecal tests (in 30 months) among individuals, by jurisdiction, January 1, 2017 – June 30, 2019
Text description and footnotes
Program participation rates range between 18.3% in New Brunswick to 54.8% in Saskatchewan, although no jurisdiction is reaching the 60% target.
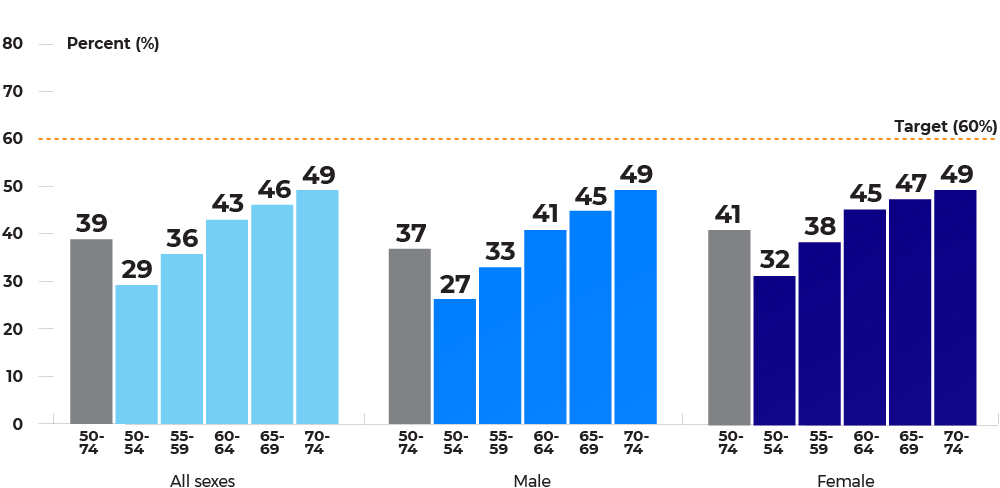
Population-based participation rates for fecal tests (in 30 months), by age group and sex, jursidictions combined, January 1, 2017 – June 30, 2019
Text description and footnotes
Participation in colorectal cancer screening is higher in older populations, with 70-74 year-olds having the highest participation. Females have higher participation than males in all age groups until age 70 – 74 where participation rates become more similar.
2. Screening program participation rate (participation rate among those invited to screen)
Why is this important? Many colorectal cancer screening programs send invitations to eligible people to participate in screening. Each province and territory has a different approach to inviting people for colorectal cancer screening. Measuring the participation rate of those invited to screen can tell us if a program’s efforts to invite people to screening are effective.
Indicator definition: The percentage of people who received an invitation from the program to participate in screening who successfully completed at least one fecal test within the measurement timeframe.
Indicator calculation:
Numerator: Number of people who successfully completed more than one fecal test in the program in a 30-month period.
Denominator: Number of people who were sent an invitation within the measurement timeframe.
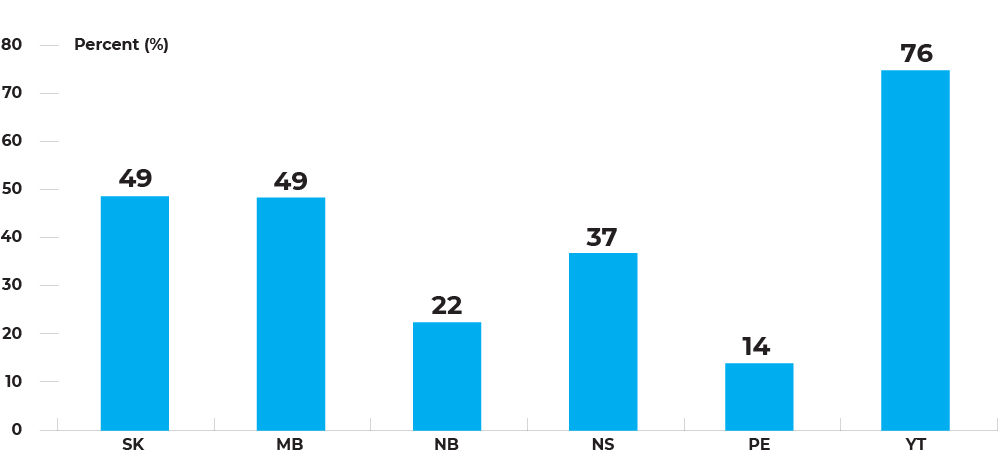
Invitation-based participation rates for fecal tests (in 30 months) among individuals, by jurisdiction, January 1, 2017 – December 31, 2018
Text description and footnotes
Invitation-based participation rates range between 14.0% in Prince Edward Island to 75.7% in Yukon.
3. Percentage overdue for colorectal cancer screening
Why is this important? Measuring the population that is overdue for colorectal cancer screening provides a better picture of how many people are being screened in the population as it also includes people who have been screened through colonoscopy or flexible sigmoidoscopy.
Indicator definition: The percentage of eligible people who are overdue for colorectal cancer screening in each calendar year.
Indicator calculation:
Numerator: Number of people who were overdue for colorectal cancer screening at the end of the measurement timeframe.
- People were considered overdue for colorectal screening if they did not have a fecal test within the last 2 years, did not have a colonoscopy within the last 10 years, and did not have a flexible sigmoidoscopy within the last 10 years.
Denominator: Number of people who are eligible for colorectal cancer screening in the calendar year.
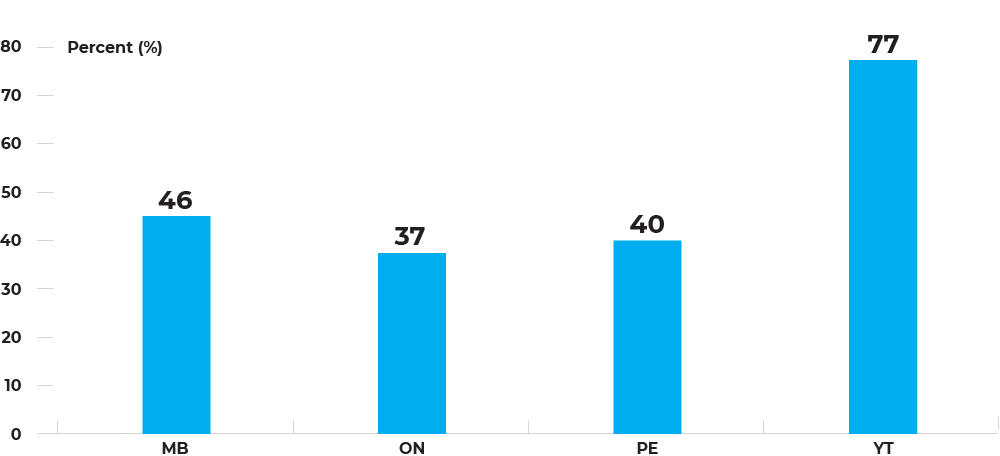
Percentage of overdue for colorectal cancer screening among individuals aged 50-74, by jurisdiction, 2018
Text description and footnotes
The percentage of people overdue for colorectal cancer screening ranged from 37.3% in Ontario to 77.1% in Yukon.
Additional considerations: Overdue for screening is the complement of up-to-date for screening. Up-to-dateness is 62.7% in Ontario, 59.9% in Prince Edward Island, 53.9% in Manitoba and 22.9% in Yukon. To fully realize the positive impacts of colorectal cancer screening programs, it is important to improve screening uptake.
Reference
1 – Walker, M.J., Meggatto, O., Gao, J., Espino-Hernandez, G., Jembere, N., et al. Measuring the impact of COVID-19 pandemic on organized cancer screening and diagnostic follow-up care in Ontario, Canada: A provincial, population-based study. Preventive Medicine: 2021. DOI: https://doi.org/10.1016/j.ypmed.2021.106586.
